PD-1 & PD-L1 and Mesothelioma Explained
PD-1 and PD-L1 inhibitors are two types of immunotherapy drugs that can be used to treat mesothelioma, an aggressive cancer caused by asbestos. They are also known as anti-PD-1 and anti-PD-L1 medications and work by turning off specific proteins that cancer cells can use to hide from the immune system.
PD-1 and PD-L1 inhibitors for mesothelioma include:
- Atezolizumab (Tecentriq®)
- Avelumab (Bavencio®)
- Durvalumab (Imfinzi®)
- Nivolumab (Opdivo®)
- Pembrolizumab (Keytruda®)
A 2025 study of more than 1,300 people with pleural mesothelioma found that PD-1 and PD-L1 inhibitors helped patients live longer than chemotherapy. About 6 in 10 people were still alive after one year, and many had their cancer shrink or stop growing.
Key Facts on PD-1 & PD-L1 and Mesothelioma
- Types of mesothelioma treated: Pleural mesothelioma (which develops in the lining of the lungs) and peritoneal mesothelioma (which affects the abdominal lining)
- When PD-1 and PD-L1 inhibitors are used: Typically given through an intravenous (IV) injection and combined with other treatments like chemotherapy and surgery
- Potential side effects: Constipation, cough, diarrhea, fatigue, nausea, skin rash, and other flu-like symptoms
- Patient eligibility factors: Cancer type, stage, and overall health
Get our Free Immunotherapy Guide to learn how PD-1/PD-L1 inhibitors and other immunotherapy treatments could help you live longer.
How Do PD-1 and PD-L1 Inhibitors Work?
Anti-PD-1 and anti-PD-L1 medications belong to a specific class of immunotherapy drugs known as immune checkpoint inhibitors. They work by blocking proteins that mesothelioma cells use to hide from T cells. The immune system makes T cells to destroy cancer.
The proteins targeted include:
- PD-1 (programmed cell death protein 1), a protein found on T cells (T lymphocytes)
- PD-L1 (programmed death ligand 1), a protein is made by cancer cells
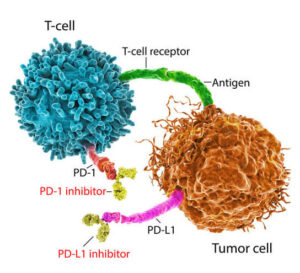
T cells use PD-1 to find out whether a cell is healthy or harmful. However, mesothelioma cells can bind PD-L1 to the PD-1 receptors on T cells. This sends an “off” signal to T cells, preventing them from attacking cancer cells.
PD-1 and PD-L1 inhibitors block the proteins from binding to one another. This prevents mesothelioma from sneaking past the immune system and creates a cytotoxic effect, meaning more of the cancer cells can be destroyed.
Contact our registered nurses today to learn if PD-1 or PD-L1 inhibitors could help you after a mesothelioma diagnosis.
Types of PD-1 and PD-L1 Medications for Mesothelioma
There are different immunotherapies that target PD-1 and PD-L1 to treat mesothelioma. Learn about the most common medications below.
PD-1 Inhibitors
PD-1 inhibitors prevent the PD-1 found on T cells from binding to PD-L1 on mesothelioma cells. Two notable PD-1 checkpoint inhibitors for mesothelioma are Keytruda and Opdivo.
Nivolumab (Opdivo®)
The U.S. Food and Drug Administration (FDA) has approved Opdivo to treat malignant pleural mesothelioma in combination with ipilimumab (Yervoy®), another class of immunotherapy drug. Yervoy blocks a different protein called CTLA-4 that mesothelioma cells can use to hide from T cells.
When given as an initial first-line treatment, Opdivo and Yervoy helped pleural mesothelioma patients live 4 months longer than those treated with chemotherapy drugs like pemetrexed and cisplatin in the phase III Checkmate 743 trial.
Pembrolizumab (Keytruda®)
The FDA has approved Keytruda as an initial treatment for mesothelioma. This approval came in September 2024 after clinical trials showed it helped mesothelioma patients live longer when combined with chemotherapy.
Trials continue to show that Keytruda can treat the two most common types of mesothelioma:
- Peritoneal mesothelioma: When given Keytruda, peritoneal mesothelioma patients had an average life expectancy of 20.9 months, according to a March 2023 study in Oncology.
- Pleural mesothelioma: A December 2023 study in The Lancet found that pleural mesothelioma patients lived 17.3 months on average when treated with Keytruda and chemotherapy.
According to a 2024 Frontiers in Oncology case study, a 67-year-old pleural mesothelioma patient went into complete remission after receiving third-line immunotherapy with Keytruda. Despite earlier treatments with surgery and chemotherapy showing limited results, he has survived more than 7 years since his diagnosis.
PD-L1 Inhibitors
PD-L1 inhibitors block cancer cells from using the PD-L1 they create to bind to T cells and turn off the body’s immune response.
The three main PD-L1 inhibitors for mesothelioma are Tecentriq, Bavencio, and Imfinzi. Currently, these medications are only available in clinical trials.
Atezolizumab (Tecentriq®)
Tecentriq has been shown to help peritoneal mesothelioma patients live longer when used in combination with bevacizumab (Avastin®).
Results of a 2021 study released in Cancer Discovery found that:
- The overall 1-year overall survival rate of patients treated with Tecentriq and Avastin was 85%. This means that more than 8 in 10 patients were still alive a year after treatment.
- The 1-year progression-free survival rate was 61%, meaning the cancer stopped growing for a year in more than 6 out of 10 patients.
A current National Cancer Institute (NCI) trial is studying if Tecentriq can help peritoneal mesothelioma patients who are also receiving chemotherapy and surgery live longer. This study is expected to be finished in 2025.
Avelumab (Bavencio®)
Bavencio is made by the company EMD Serono. It’s currently approved to treat cancers like urothelial carcinoma. Doctors are studying how it can help mesothelioma patients in clinical trials.
A 2023 study published in JTO Clinical and Research Reports found that mesothelioma patients lived over 1 year on average when treated with Bavencio and radiation therapy.
Every patient in the study had already been treated but had relapsed (meaning their cancer came back) before starting Bavencio.
Durvalumab (Imfinzi®)
Imfinzi is manufactured by AstraZeneca. Many studies show how Imfinzi can help mesothelioma patients, particularly when used along with another immunotherapy called tremelimumab (Imjudo®).
These include:
- Baylor College of Medicine trial: Thoracic oncology (cancer) doctors are studying whether Imfinzi, Imjudo, and chemotherapy will help mesothelioma patients live longer than those treated with just Imfinzi and Imjudo. The results are expected in 2028.
- NIBIT-MESO-1 trial: Pleural and peritoneal mesothelioma patients had an average life expectancy of 16.6 months when treated with a combination of Imfinzi and Imjudo.
- PrE0505 trial: Pleural mesothelioma patients lived 20.4 months on average when treated with Imfinzi and chemotherapy.
Get our Free Immunotherapy Guide to see how checkpoint inhibitors for mesothelioma can help you or a loved one.
How Are PD-1 and PD-L1 Inhibitors Used to Treat Mesothelioma?
PD-1 and PD-L1 inhibitors are often used in combination with other mesothelioma treatments to help patients live longer.
Many studies have demonstrated that multimodal therapy (the use of two or more treatments) improves survival compared to monotherapy (where only one PD-1 or PD-L1 inhibitor is used).
Doctors can use PD-1 and PD-L1 inhibitors with:
- Chemotherapy: Cancer-killing medication can shrink tumors, while T cells fight mesothelioma cells. Combining these treatments can be very helpful for mesothelioma that can’t be removed with surgery (known as unresectable cancer).
- Other immunotherapies: Combining PD-1/PD-L1 inhibitors with other cancer immunotherapy drugs can enhance the immune response by targeting different immune checkpoints simultaneously.
- Radiation: Beams of energy shrink tumors while PD-1 and PD-L1 inhibitors help T cells kill mesothelioma cells.
- Surgery: Doctors remove cancer tumors from the body, and PD-1 and PD-L1 inhibitors allow the immune system to kill remaining cancer cells.
Which treatments will be used in your particular case depends on factors like the type of mesothelioma you have, how far it has spread, and your overall health.
Clinical Trials for PD-1 & PD-L1 and Mesothelioma
Doctors across the country are conducting mesothelioma clinical trials on PD-1 and PD-L1 inhibitors.
The goal of this research is to see how these medications can more effectively treat mesothelioma, depending on unique patient characteristics such as cancer type and stage.
Some ongoing mesothelioma PD-1 and PD-L1 inhibitor trials include:
- DREAM3R trial: Doctors are investigating whether combining the PD-1 inhibitor Imfinzi with standard chemotherapy can improve overall survival.
- Kimmel Cancer Center Opdivo trial: Researchers are studying how Opdivo with or without Yervoy could help pleural mesothelioma patients before undergoing surgery. This phase II trial is expected to be completed in 2026.
- REGOMUNE trial: Researchers are trying to find out whether Bavencio could help patients with metastatic (advanced) mesothelioma or other solid tumors live longer. Patients will receive Bavencio and another medication known as regorafenib (Stivarga®).
Many of these are multicenter clinical trials, meaning several cancer centers across the country are enrolling eligible patients.
Use our Free Doctor Match to find specialists near you who can determine if you qualify for immunotherapy clinical trials.
Side Effects of PD-1 and PD-L1 Inhibitors for Mesothelioma
PD-1 and PD-L1 inhibitors can cause side effects because T cells may kill healthy cells in addition to cancer cells.
Common side effects of PD-1/PD-L1 inhibitors include:
- Cough
- Diarrhea
- Fatigue
- Muscle and joint pain
- Nausea
- Skin rash
Some patients may experience more severe or rare side effects, like autoimmune reactions that make it harder to treat mesothelioma.
Your health care team will monitor you for potential side effects of PD-1 or PD-L1 mesothelioma treatments and can help manage them as they arise.
What to Expect During PD-1 and PD-L1 Treatment Sessions
Here’s what you can expect while undergoing PD-1 and PD-L1 treatment for mesothelioma:
1. Receive PD-1 and PD-L1 Inhibitor Infusions
You’ll typically receive PD-1 and PD-L1 inhibitor infusions in a single arm through an IV. The infusions are given in cycles, meaning you’ll receive one every 2-6 weeks and then have a rest period so your body can recover.
Your infusion schedule may vary based on your treatment plan, mesothelioma stage, and overall health.
2. Watch for Potential Drug Reactions and Side Effects
The main goal of PD-1 and PD-L1 inhibitor treatment is to destroy as much of the cancer as possible. However, you could experience side effects because these treatments will cause your immune system to kill both healthy and bad cells.
Your doctors will closely monitor you for any adverse events (side effects) and help manage them.
Be sure to tell a member of your medical team if you start to feel unwell. They can recommend medications and other options to reduce uncomfortable side effects.
3. Monitor Cancer and Adjust Treatments
Your doctors will monitor how your mesothelioma tumors respond during and after treatment.
This includes:
- Conducting imaging tests to monitor tumor size and spread
- Noting changes in mesothelioma symptoms
- Performing blood tests to check for biomarkers (signs that cancer is present)
If your tumors don’t respond to PD-1 or PD-L1 inhibitors for mesothelioma, your cancer care team can adjust your treatment plan to include other therapies that could help you live longer.
How Much Do PD-1 and PD-L1 Inhibitors Cost?
The cost of PD-1 and PD-L1 treatment varies depending on the drug(s) used, your insurance plan, and other factors.
Here’s a cost breakdown by the type of inhibitor:
- Opdivo: Without insurance, one infusion of Opdivo and Yervoy costs $28,292. You’ll most likely need to get more than one infusion.
- Keytruda: An infusion of Keytruda costs $11,337.36 before insurance.
- All other PD-1 and PD-L1 inhibitors: You can only access the other inhibitors through clinical trials, which are free to join. However, you must meet specific criteria to enroll in a study.
Call (866) 608-8933 now to learn how we can help you pursue financial assistance for mesothelioma PD-1 and PD-L1 treatments.
Learn if PD-1 or PD-L1 Inhibitors for Mesothelioma Are Right for You
PD-1 and PD-L1 inhibitors show promise in helping mesothelioma patients live longer.
With some inhibitors like Opdivo and Keytruda already approved and more being studied in clinical trials, the hope is that these immunotherapies will give patients more time to spend with their families. In some cases, PD-1 and PD-L1 inhibitors could even send mesothelioma into remission.
If you or a loved one was recently diagnosed with mesothelioma, we can connect you with doctors who can see if PD-1 and PD-L1 inhibitors are a good fit for your case.
Use our Free Doctor Match to find immunotherapy cancer specialists near you right now.
PD-1 & PD-L1 and Mesothelioma FAQs
What is PD-1 in mesothelioma?
PD-1 is a protein made by your immune system’s T cells. The T cells use PD-1 to determine which cells are healthy and which cells are harmful.
In cases of mesothelioma, tumor cells make their own protein called PD-L1. Mesothelioma binds PD-L1 to the PD-1 of T cells. This prevents the T cells from destroying the mesothelioma cells.
Doctors can use immunotherapy drugs called PD-1 and PD-L1 inhibitors to prevent this binding process from happening. With the addition of these medications, your T cells can destroy more cancer cells.
What is the best immunotherapy for mesothelioma?
Some of the best immunotherapies for mesothelioma are PD-1 and PD-L1 inhibitors.
The anti-PD-1 drugs nivolumab (Opdivo®) and pembrolizumab (Keytruda®) are both FDA-approved to treat mesothelioma. Other PD-1 and PD-L1 inhibitors are showing success in clinical trials.
The best immunotherapy drugs for your case will depend on the type and stage of your cancer, your overall health, and other factors.
Contact our team today for help finding the best mesothelioma immunotherapy treatment options for you or a loved one.
What is the success rate of immunotherapy for mesothelioma?
PD-L1 and PD-1 immunotherapy drugs can often very successfully treat mesothelioma patients. In the CheckMate 743 trial testing Opdivo with ipilimumab (Yervoy®), patients who received immunotherapy lived for 18.1 months — 4 months longer than those treated with chemotherapy.
Other PD-1 & PD-L1 and mesothelioma trials have shown how these inhibitors can help patients live longer.
How long do side effects from PD-1 or PD-L1 drugs last?
The length of any side effects or toxicity from PD-1 or PD-L1 inhibitors varies from patient to patient. Common side effects like fatigue, rash, nausea, and diarrhea typically resolve within a few weeks.
For more serious side effects, including immune-related reactions, doctors may need to pause or stop using PD-1 or PD-L1 inhibitors.
How long does PD-1/PD-L1 treatment for mesothelioma last?
The duration of PD-1/PD-L1 mesothelioma treatment can vary based on several factors, including the patient’s response to treatment, their cancer stage, and the specific treatment plan prescribed by their oncologist.
Some patients may undergo limited treatment for a few weeks or months. Others may continue treatment for 1-2 years or longer if they are still responding well to the drug(s) and not experiencing significant side effects.
What cancers can PD-1 and PD-L1 inhibitors treat?
The PD-1 inhibitors Opdivo and Keytruda are currently approved to treat pleural mesothelioma.
Other PD-1 and PD-L1 inhibitors like atezolizumab (Tecentriq®), avelumab (Bavencio®), and durvalumab (Imfinzi®) are being used to treat pleural and peritoneal mesothelioma in clinical trials.
These medications can also be used to treat small cell and non-small cell lung cancer, melanoma, carcinoma, and other cancers.





