What Is Mesothelioma Immunotherapy?
Mesothelioma immunotherapy is a type of cancer treatment that enhances the immune system’s ability to identify and destroy tumor cells. It can help slow down the cancer, improve quality of life, and extend survival in some cases.
Depending on their individual treatment plan, patients may receive immunotherapy for mesothelioma on its own or in combination with chemotherapy or surgery.
There are several types of immunotherapy used to treat mesothelioma, with immune checkpoint inhibitors being the most common. These drugs “unblock” the immune system so it can fight cancer.
Other promising forms of immunotherapy include:
- Adoptive cell therapies
- Monoclonal antibodies
- Vaccines
Immunotherapy isn’t right for everyone, but it may help some patients live longer and feel better. This is especially true for those who can’t get surgery or whose cancer has spread from where it started.
Key Facts on Immunotherapy for Mesothelioma
- It’s a systemic treatment, meaning it works throughout the entire body to kill cancer cells.
- It’s now recommended as a first-line (initial) treatment for patients with inoperable mesothelioma.
- Researchers are testing emerging forms of immunotherapy like CAR T-cell therapy and cancer vaccines in clinical trials.
Download our Free Mesothelioma Immunotherapy Guide to learn more about this treatment and whether it could help you or a loved one.
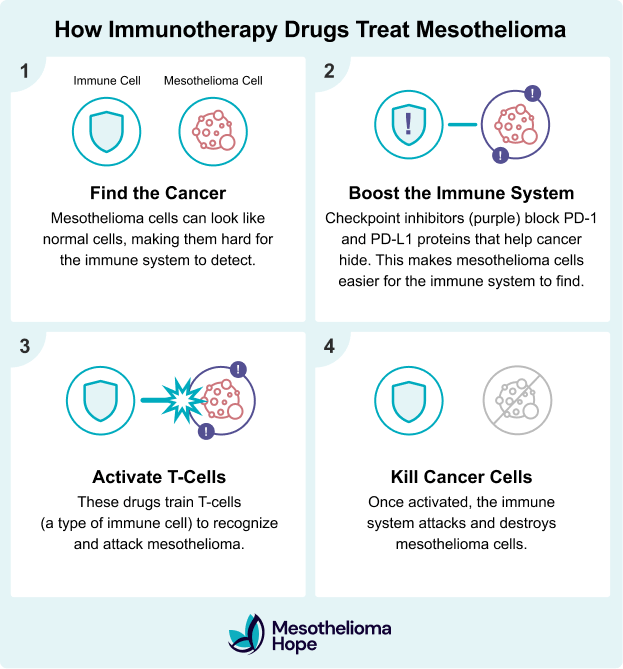
How Does Immunotherapy Work for Mesothelioma?
Immunotherapy improves the immune system’s ability to find and attack mesothelioma cancer. Mesothelioma cells can sneak past the body’s natural immune defenses. Immunotherapy helps overcome this by training immune cells to recognize the cancer.
Immunotherapy works in two main ways:
- Strengthens the immune system so it can better fight cancer cells
- Finds parts of the cancer that help it “hide” from the immune system
In addition to immunotherapy, scientists are developing targeted therapies that attack specific genes or proteins that help mesothelioma grow or spread. These advances hold promise for improving life expectancy in hard-to-treat cases.
Mesothelioma Immunotherapy vs. Chemotherapy
Immunotherapy and chemotherapy both play important roles in treating mesothelioma, but they work in different ways. Chemotherapy uses drugs to directly attack and kill cancer cells, while immunotherapy works by helping the immune system do that job more naturally and with greater precision.
In the CheckMate 743 clinical trial, patients with inoperable pleural mesothelioma who received the immunotherapy drugs nivolumab (Opdivo®) and ipilimumab (Yervoy®) lived longer than those who received chemotherapy alone. Many also experienced fewer side effects.

“Immunotherapy is like giving your body an extra set of tools to combat the disease more effectively.”
FDA-Approved Mesothelioma Immunotherapy Drugs
Three immunotherapy drugs have received approval from the U.S. Food and Drug Administration (FDA) as treatments for mesothelioma: Opdivo, Yervoy, and Keytruda. These options give doctors more flexibility to tailor care to each patient’s diagnosis.
Opdivo With Yervoy
In 2020, the FDA approved Opdivo + Yervoy as the first immunotherapy combination for mesothelioma. This marked a major turning point in mesothelioma care.
These immune checkpoint inhibitors block proteins (PD-1 and CTLA-4) that cancer cells use to hide from the immune system, allowing the body to launch a more targeted attack.
The approval was based on the results of the CheckMate 743 trial, which showed a significant improvement in survival compared to chemotherapy. Patients who received Opdivo and Yervoy lived a median of 18.1 months, compared to 14.1 months for those receiving chemotherapy alone. The benefit was especially strong for patients with fast-growing cell types like sarcomatoid mesothelioma.
Keytruda
In 2024, pembrolizumab (Keytruda®) was approved for use with chemotherapy as a first-line treatment of advanced pleural mesothelioma. The approval was based on results that showed Keytruda improved the body’s immune response and made chemotherapy more effective.
Keytruda is already well-known for its success in treating other cancers, and its approval for mesothelioma represents another important step forward in expanding treatments for this rare cancer.
Use our Free Doctor Match to get connected with local experts who can guide you through your options for mesothelioma immunotherapy.
Emerging Immunotherapies for Mesothelioma
Researchers are working hard to test new types of immunotherapy that go beyond current FDA-approved medications. Even though most of these emerging treatments are still in clinical trials, early results are encouraging and may lead to higher survival rates.
Adoptive Cell Therapies
Adoptive cell therapy involves collecting a patient’s immune cells (usually T cells) and modifying them to make them better at fighting mesothelioma. The most well-known example is CAR T-cell therapy.
A May 2025 OncLive report outlined an early-stage clinical trial at Memorial Sloan Kettering Cancer Center that investigated a combination of CAR T-cell therapy and Keytruda in patients with pleural mesothelioma.
The results were promising:
- 83% of patients were still alive 1 year after treatment
- 2 patients had no signs of active cancer on imaging scans
- 8 patients had stable disease for at least 6 months, meaning their cancer didn’t grow or spread
More research is being done to confirm these results in larger groups of patients. However, these early findings suggest that combining CAR T-cell therapy with immune checkpoint inhibitors could become a powerful new option for treating mesothelioma.
Monoclonal Antibodies
Monoclonal antibodies (mAbs) are a type of immunotherapy made in a lab to act like natural antibodies in the immune system. They recognize and attach to specific substances (antigens) on mesothelioma cells to block cancer growth, deliver cancer-killing drugs, or flag the cells so the immune system can destroy them.
A promising mAb called BNT327 targets mesothelin, a protein found on many mesothelioma cells.
In a study of 31 patients with advanced mesothelioma:
- About 52% had their tumors shrink
- Over 90% saw their cancer shrink or stop growing
- Participants lived over a year without their cancer getting worse
The treatment appeared to be more effective for peritoneal mesothelioma, which develops in the lining of the abdomen.
Vaccines
Mesothelioma vaccines work by helping the immune system recognize cancer cells more effectively. They are currently only available in clinical trials.
Vaccines being tested for mesothelioma include:
- Galinpepimut-S (GPS), a vaccine that targets the Wilms Tumor 1 (WT1) protein commonly found on mesothelioma cells. A trial for this vaccine has been approved by the FDA but hasn’t started accepting patients.
- ONCOS-102, a vaccine that shows promise in combination with chemotherapy.
- Poly-ICLC, a synthetic vaccine also known as Hiltonol® that’s injected into tumors before surgery.
- UV1, a vaccine that targets a known sign of cancer called telomerase.
These vaccines are part of promising research that could give mesothelioma patients better treatment options.
Benefits of Immunotherapy in Mesothelioma Treatment
The main benefit of immunotherapy for mesothelioma is that it can help improve life expectancy in certain patients. This type of treatment may be especially helpful for patients whose cancer has spread or returned after other treatments.
Other benefits of mesothelioma immunotherapy include:
- Fewer and milder side effects compared to chemotherapy in many cases
- Improved chance of mesothelioma remission (where signs of cancer lessen or disappear)
- Ongoing immune response that may continue working after treatment ends
- Tumor reduction and delayed or slowed cancer spread
These advantages can make a big difference in daily life for people living with mesothelioma. Many patients report feeling stronger and more active during immunotherapy compared to chemotherapy.
Talking with a mesothelioma specialist can help you find out if immunotherapy is a good fit based on your health and diagnosis. As doctors continue to study the benefits of immunotherapy, more patients may be able to access this treatment and become mesothelioma survivors.

“I have some anecdotal evidence of people who’ve been treated just with immunotherapy who had near complete resolution of their tumors for an extended period of time without any other intervention. It’s been quite remarkable.”
Who’s Eligible for Mesothelioma Immunotherapy?
Not every patient is a candidate for immunotherapy, but it may be a strong option depending on your diagnosis, overall health, and treatment history. Your doctor can help determine if this type of treatment fits into your care plan.
Immunotherapy may be recommended:
- Shortly after diagnosis, especially for late-stage mesothelioma
- If surgery isn’t an option, either due to tumor location or other health issues
- As part of a multimodal treatment plan that includes chemotherapy or radiation
- If other treatments have stopped working, or if the cancer comes back
Some patients may also qualify to join clinical trials to access newer immunotherapy drugs that aren’t available to the public yet.
Immunotherapy Mesothelioma Success Rate
Overall, mesothelioma life expectancy with immunotherapy is steadily improving. While everyone’s experience varies, newer therapies and combinations are leading to longer survival for many patients.
About 23% of pleural mesothelioma patients were still alive after 3 years (a figure known as the survival rate). Even more impressive, 8 of the patients had their cancer go into complete remission.
Immunotherapy for peritoneal mesothelioma has also shown success. A 2024 Cureus report highlighted a patient who went into remission after 2 years of treatment with Keytruda and was still alive over 4 years later.
What Is the Life Expectancy of Someone With Mesothelioma Immunotherapy?
Life expectancy for someone receiving immunotherapy for mesothelioma depends on several factors, including the cancer’s stage, the cell type, and the patient’s overall health.
On average, patients treated with immunotherapy live 18 to 24 months or longer. Some patients have lived well beyond this range with new combinations of treatments.
While outcomes vary, immunotherapy has led to longer survival times than chemotherapy in many cases, with fewer side effects. A small number of patients have even seen their cancer go into complete remission.
Immunotherapy for Mesothelioma Side Effects
Some people may experience side effects from mesothelioma immunotherapy, though not everyone does. The specific treatments and your overall health can affect which side effects you experience and how severe they are.
Common side effects of immunotherapy for mesothelioma include:
- Changes in appetite
- Constipation or diarrhea
- Fatigue
- Joint pain
- Mouth soreness or irritation
- Nausea
- Skin irritation, rash, or itching
Your medical team will monitor you closely for side effects during immunotherapy. They may prescribe medications to help with nausea or use steroids to reduce inflammation. If you have a serious reaction to a drug, your doctor may have you stop taking it.
Download our Free Immunotherapy Guide to learn more about possible side effects and how to manage them.
How Much Does Immunotherapy for Mesothelioma Cost?
The cost of mesothelioma immunotherapy with Opdivo and Yervoy can exceed $292,000 a year, according to an article in Frontiers in Oncology. Reuters also notes it costs an average of $150,000 per year for checkpoint inhibitors like Keytruda and Imfinzi.
The Mesothelioma Hope team understands that the high costs of immunotherapy and other treatments can be a significant burden for cancer patients. We’re committed to helping you navigate the financial challenges of a mesothelioma diagnosis.
Contact our Patient Advocates to learn about mesothelioma settlements, asbestos trust fund claims, and other ways to access compensation for your medical treatment.
Immunotherapy and the Future of Mesothelioma Treatment
In early 2025, the American Society of Clinical Oncology (ASCO) updated its official treatment guidelines to include immunotherapy combined with chemotherapy as a standard treatment for unresectable pleural mesothelioma (cancer that can’t be removed with surgery).
Researchers continue to study innovative ways to harness the power of the immune system to fight this aggressive cancer.
Chemotherapy With Immunotherapy for Mesothelioma
Combining immunotherapy with chemotherapy drugs like pemetrexed has shown increasingly promising results in recent trials.
The phase II DREAM trial, using durvalumab (Imfinzi®) plus chemotherapy, reported a median overall survival (OS) of 20.4 months, much higher than the 12 months seen with chemotherapy alone. Patients with epithelioid pleural mesothelioma lived even longer, around 24.3 months.

“I think there will be an immunotherapy component for pretty much everything at some point.”
Surgery With Immunotherapy for Mesothelioma
Doctors have been looking into whether combining mesothelioma surgery with immunotherapy could help patients live longer. The idea is to remove as much of the cancer as possible, then use the immune system to help fight off anything left behind.
But a major study found that surgery didn’t lead to better results. In fact, patients who had surgery often faced more complications and a harder recovery.
Right now, immunotherapy— on its own or with other treatments — is usually a safer and more effective option. Some clinical trials are still exploring whether adding surgery might help, but for now, this approach is mostly used in research settings where patients are closely watched.
Get Help Accessing Mesothelioma Immunotherapy
When it comes to mesothelioma immunotherapy, having the right medical team by your side is crucial. Mesothelioma Hope can help you find a local medical oncologist who specializes in immunotherapy.
We can also help you:
- Connect with other patients receiving immunotherapy
- Learn what to expect from mesothelioma immunotherapy
- Pursue compensation for treatment, travel, and out-of-pocket expenses
Call us now at (866) 608-8933 or get your Free Immunotherapy Guide to learn more about how we can support you or someone you love.
Immunotherapy and Mesothelioma FAQs
Is immunotherapy effective with mesothelioma?
Yes, immunotherapy has become one of the most promising treatments for mesothelioma, particularly pleural mesothelioma.
Drugs like nivolumab (Opdivo) and ipilimumab (Yervoy) have helped many patients by slowing tumor growth and improving quality of life. In some trials, immunotherapy proved more effective than chemotherapy, particularly in those with sarcomatoid or biphasic mesothelioma, which are the more aggressive mesothelioma cell types.
Cancer survivor Ellen Patton received immunotherapy early in her treatment after being diagnosed with pleural mesothelioma (cancer of the lung lining). She is still alive more than 24 years later, showing the long-term potential of immunotherapy for mesothelioma.
Get our Free Mesothelioma Survivors Guide for more stories about patients who are thriving many years after their diagnosis and the treatments that helped them.
How does cell type affect the response to mesothelioma immunotherapy?
The type of mesothelioma cell can affect how well a person responds to immunotherapy. There are three main types of mesothelioma cells: epithelioid, sarcomatoid, and biphasic (which is a mix of the first two).
- Sarcomatoid and biphasic types usually do not respond well to chemotherapy. But studies show that these types may respond better to immunotherapy. So, for people with sarcomatoid or biphasic mesothelioma, immunotherapy could work better than traditional chemotherapy.
- Epithelioid mesothelioma is the most common type and usually responds fairly well to chemotherapy. In this case, immunotherapy and chemotherapy seem to give similar results, so either treatment could be used.
What are the side effects of immunotherapy for pleural mesothelioma?
Common side effects of immunotherapy for pleural mesothelioma include fatigue, rash, diarrhea, and joint pain. These usually happen because immunotherapy boosts your immune system, which can sometimes cause inflammation in healthy cells.
Immunotherapy side effects tend to be milder than those of chemotherapy, but they can still affect your daily life.
In rare cases, people may develop more serious side effects affecting the lungs, liver, or other organs. Most of the time, these problems can be managed if caught early. That’s why it’s important to stay in close contact with your medical team during treatment and report any new symptoms right away.
Can mesothelioma be treated with immunotherapy?
Yes, mesothelioma can be treated with immunotherapy, particularly with checkpoint inhibitors like Opdivo and Yervoy. Research has shown that this mesothelioma immunotherapy combination not only improves survival but also helps maintain quality of life.
What is the success rate of immunotherapy for mesothelioma?
In clinical trials, around 40% of patients with epithelioid mesothelioma responded to immunotherapy, meaning their tumors shrank or stopped growing.
Response rates were similar to chemotherapy, but survival outcomes were better, especially in patients with non-epithelioid cell types, such as sarcomatoid or biphasic.
About 1 in 4 patients (around 23–25%) treated with immunotherapy were still alive 3 years later, a significant improvement over past treatments.
Can immunotherapy cure mesothelioma?
Immunotherapy is not considered a cure for mesothelioma, but it has helped some patients live significantly longer or go into complete remission. If this happens, the cancer becomes undetectable and may stay that way for years.
Researchers are still learning why certain patients respond better than others. For now, immunotherapy offers hope for longer survival and better quality of life, especially when started early or combined with other treatments.
How effective is immunotherapy for mesothelioma?
Immunotherapy, particularly Opdivo + Yervoy, has demonstrated meaningful benefits for mesothelioma. Median overall survival increased from 14.1 to 18.1 months in the CheckMate 743 clinical trial, and 3-year survival nearly doubled from 15% to 23–25%.
These outcomes show immunotherapy can be a powerful and effective option in certain mesothelioma treatment plans.






